Regeneration of the embryonic heart
Regeneration of the embryonic heart
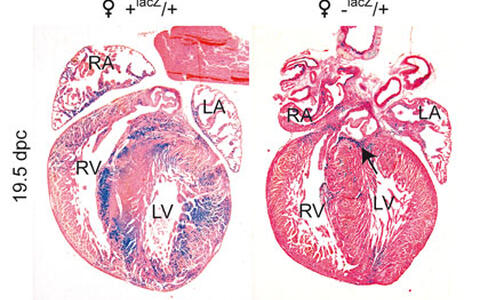
Figure 1. ß-Galactosidase staining of 19.5 dpc (days post coitum) foetal hearts prior to birth (counterstained with Eosin). The control heart (♀ +lacZ/+, heterozygous for an X-linked lacZ reporter gene) shows large patches of ß-Gal positive and negative cells due to random X chromosome inactivation. In the heterozygous Hccs-knock-out heart (♀ -lacZ/+) the lacZ reporter gene is linked to the X chromosome carrying the defective Hccs gene allowing the detection of Hccs deficient cells by ß-Gal staining. Due to cardiac regeneration during embryonic development the proportion of Hccs deficient cells is minimized until birth. Very few isolated Hccs deficient cardiomyocytes can be detected within the ventricular and atrial myocardium with only the basal region of the interventricular septum showing a larger cluster of ß-Gal positive cells (see arrow). RA = right atrium, LA = left atrium, RV = right ventricle, LV = left ventricle.
The regeneration of functional myocardium after cardiac injury is currently one of the most rapidly developing fields in cardiovascular research. Several different approaches based on injection of various cell types as well as mobilisation or stimulation of endogenous cells to repair the damaged heart have been reported. Despite lots of promising and encouraging findings some controversial or inconclusive results have highlighted the need for a better understanding of the molecular and cellular mechanisms underlying a solid myocardial regeneration. In this regard, the embryonic heart is a perfect model to study these processes as key events like cardiomyocyte proliferation, differentiation from stem or progenitor cells as well as regional and functional specification occur physiologically in the developing heart.
Our recent findings have shown that the embryonic murine heart has a remarkable regenerative capacity. We have inactivated the X-linked gene encoding Holocytochrome c synthase (Hccs), an enzyme responsible for activation of cytochromes c and c1 within the mitochondrial electron transport chain, specifically in the developing mouse heart. Loss of Hccs activity results in cellular energy starvation causing disturbed cardiomyocyte differentiation and ultimately cellular degeneration. In contrast to the observed mid-gestational lethality of hemizygous Hccs knock-out (KO) males, heterozygous females appeared normal during the first months of life with surprisingly few clusters of affected cardiomyocytes, considering an expected mosaic of affected and normal cardiomyocytes as a result of random X chromosome inactivation. However, analyses of heterozygous female embryos revealed the expected 50:50 ratio of Hccs deficient to normal cardiac cells at mid-gestation with a progressive reduction in disease tissue to 10% prior to birth. We could show that this significant change is accounted for by increased proliferation of remaining healthy cardiac cells. These data reveal a previously unrecognised but impressive regenerative capacity of the mid-gestational heart that can compensate for an effective loss of at least 50% of cardiac tissue to enable formation of a functional heart at birth.
The detailed characterisation of molecular signalling pathways as well as the identification of cell types involved in embryonic heart regeneration are currently underway and should provide major new insights into heart development and cardiac signalling. Furthermore, the identification of regenerative factors and stimuli within the embryonic heart will improve the understanding of cardiac regeneration in the adult heart and potentially enable the development of new therapeutic strategies for cardiac repair after injury.
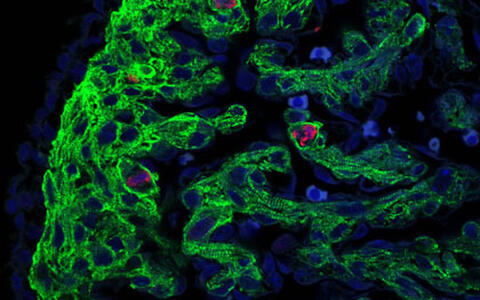
Figure 2. Immunofluorescence image obtained with a confocal laser scanning microscope showing proliferating cells in the ventricular myocardium of a 13.5 dpc (days post coitum) heterozygous Hccs KO female embryo. Cardiomyocytes are marked with an antibody against the sarcomer protein Troponin T (green) while mitotic cells are detected using an antibody against phosphorylated histone H3 (red), a specific marker for cells undergoing mitosis. Nuclei were stained blue using the DNA dye DAPI. To the left lies the ventricular compact layer surrounded by the Troponin T negative epicardium while to the right the trabeculated myocardium is visible composed of elongated, cross striated cardiomyocytes and Troponin T negative endocardial cells. The image reveals that the majority of mitotic cells in the embryonic heart are indeed cardiomyocytes but not other cardiac cell types like fibroblasts, epicardial or endocardial cells.
Postnatal maturation and remodelling of the myocardium to compensate for disturbed heart development
The model for regeneration of the embryonic heart described above assumes that a morphologically normal heart can be generated from approximately 50% of healthy cardiac cells present at mid-gestation. However, detailed analyses of the perinatal period have revealed that this is not fully true. Although cardiac function in heterozygous Hccs KO females is sufficient for survival during embryogenesis and postnatal life, there are obvious signs of incomplete myocardial development. For example, heterozygous Hccs KO female foetuses immediately before birth as well as newborns show thinner ventricular walls compared to controls as well as left ventricular non-compaction (i.e. thin myocardial compact layer accompanied by hyper-trabeculation). These abnormalities appear less obvious in heterozygous females aged 8 weeks suggesting that postnatal maturation of ventricular myocardium occurs to compensate for the disturbed cardiac development as a consequence of 50% of damaged cells at mid-gestation. This postnatal “catch-up” growth of the myocardium is mainly mediated by cardiomyocyte hypertrophy which is evident from neonatal stages onward until adulthood. However, some heterozygous Hccs KO females show less hypertrophy but increased cardiomyocyte proliferation within the first week after birth suggesting that both mechanisms play a role in postnatal maturation of the myocardium.
Despite embryonic heart regeneration and postnatal myocardial maturation described above, residual clusters of respiratory chain deficient cells in postnatal and adult hearts appear to predispose some heterozygous Hccs knock-out females to various cardiomyopathies and pathologies of the cardiac conduction system, with the clinical outcome likely dependent on the position and proportion of the remaining affected cells.
Ongoing research
-
Molecular analyses of stimuli and signalling pathways involved in embryonic heart regeneration (Methods: expression microarray analyses, real-time PCR, immunofluorescence, in situ hybridisation)
-
Identification of cell types involved in embryonic heart regeneration (Methods: cell culture of embryonic heart cells, FACS, heart explant culture)
-
Molecular analyses of signalling pathways involved in postnatal myocardial maturation (Methods: expression microarray analyses, real-time PCR, immunofluorescence, cardiac stem cell techniques)
-
Evaluation of cardiac function during embryonic heart regeneration (Methods: longitudinal echocardiography studies during embryonic heart development)
-
Evaluation of the influence of remaining Hccs deficient cells on cardiac function in adult heterozygous Hccs KO females (Methods: echocardiography, cardiac stress models, ECG)
-
Studies on the consequences of late inactivation of Hccs in the foetal or adult heart using heart specific inducible Cre expressing mice
Relevant Publications:
Drenckhahn JD, Schwarz QP, Gray S, Laskowski A, Kiriazis H, Ming Z, Harvey RP, Du XJ, Thorburn DR, Cox TC (2008). Compensatory growth of healthy cardiac cells in the presence of diseased cells restores tissue homeostasis during heart development.
Developmental Cell 15 (4): 521-533.
Involved Scientists
Jörg-Detlef Drenckhahn (Postdoc)
Manuela Magarin (PhD student)
Patrick Langner (undergraduate student)
Susanne Probst (PhD student)