Electron Microscopy
Séverine Kunz
Profile
Electron microscopy (EM) is an indispensable tool in many fields of cell biology and medicine for studying subcellular structures in normal and diseased states. However, since the electron beam is only stable in a high vacuum (a vacuum with very low pressure), it is impossible to image living cells or tissues. Samples have to be chemically fixed and embedded, or immobilized through very low (cryogenic) temperatures – while retaining biological structures in a close-to-native state.
Our tools
- Transmission electron microscopes:
- Soon to come: JEM 2100 Plus (200kV), 20MP CMOS camera Xarosa with tomography set-up
- Talos L120 C (120 kV) with long duration dewar for cryo applications, 16M Ceta CMOS camera and 2 Gatan cryo holders (FEI)
- Morgagni (80 kV) with 11M Morada CCD camera (FEI)
- Leo 910 (120 kV) with 11M Quemesa CCD camera (Zeiss)
-
Scanning electron microscopes:
- Helios 5 CX (Thermo Fisher Scientific / FEI) with Elstar column and Tomahawk Gallium-Fib equipped with in-column, in-lens, retractable DBS and STEM detectors. This is a shared investment with the Leibniz Institute for Molecular Pharmacology (FMP)
- Soon to come: Helios 5 Hydra with Plasma-FIB (xenon, oxygen, argon and nitrogen)
- 4 ultramicrotomes, one with a cryo chamber for the Tokuyasu technique (UC7, Leica)
- High-pressure freezer (ICE, Leica), freeze substitution device (AFS2, Leica) and grid plunger (GP2, Leica)
- Sputter coater (CCU-010, Safematic), GloCube glow discharge system, trimming device and others.
Team
Service and Technology
Deploying the electron beam
Our group offers a set of EM methods to explore manifold specimen types spanning from human biopsies to model organisms, such as mice, zebrafish, and fruit flies. Imaging modalities range from Transmission EM (TEM) to Scanning EM of tissue blocs. The data obtained by EM offers detailed context information about the ultrastructure of cells and how organelles interact. Immuno-EM techniques or correlative light and electron microscopy (CLEM ) methods combine functional information on protein identity with the underlying morphological features at nanometer resolution.
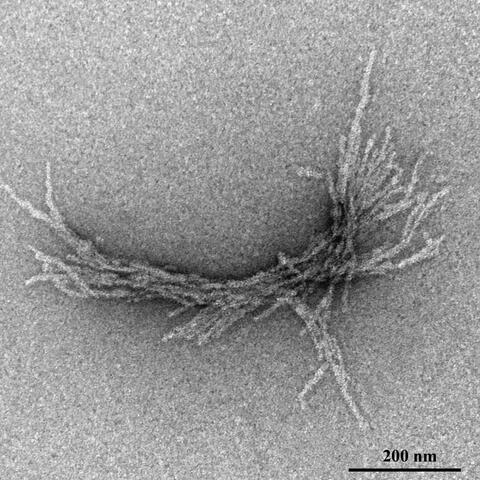
Such structures are formed in neuronal brain cells of Huntington’s disease patients. This fibril was produced in the test tube for experimental investigation. (negative contrast)
Methods
Sample preparation:
- Chemical fixation
- High-pressure freezing and freeze substitution
- Plunge freezing
Transmission EM of thin samples:
- Resin sections of small organisms, tissue and cell culture, and proteins in solution (negative stain)
- Tomography of thicker resin sections
Scanning EM of larger volumes:
- Fib-SEM
- Array tomography or serial sections
Immuno-EM:
- Tokuyasu technique of thin cryo section
- Pre-embedding techniques
CLEM:
- Pre-embedding, in-resin and other approaches
Publications
News
Outlook
A 3D PLUNGE INSIDE THE CELL
We offer several approaches to obtain high-resolution information in 3D, like tomography of sections with TEM or array tomography of serial sections by SEM. Especially suitable to resolve organelles or whole cells in 3D is FIBSEM (Focussed Ion Beam Scanning Electron Microscopy). It can be used to achieve a resolution of up to 3nm isotropic voxel on volumes of 1-20μm3. Our new device, installed in 2023, will be equipped with a powerful plasma-FIB, which is able to mill quicker and larger areas as well as improve working with CLEM-compatible resins. We will be thus able to visualize small cellular compartments, such as vesicles and mitochondria, as well as bigger cellular arrangements of complex tissue in 3D.