Customized Cell Engineering: MDCell
Helmholtz Innovation Lab
Profile
MDCell is hosting cooperation projects between the Max Delbrück Center for Molecular Medicine Berlin (MDC) and external partners. Such partners may be private companies interested in technologies developed at the MDC.
MDCell is fueled by discoveries and inventions developed in the research groups of renowned scientists in the gene and cell therapy field: Dr. Zsuzsanna Izsvák and Prof. Wolfgang Uckert.
MDCell’s focus is on the non-viral gene transfer based on the Sleeping Beauty 100x transposon system for the development of cell- and gene-therapies.
Scientific advisors
Scientific advisors of MDCell are Dr. Zsuzsanna Izsvak (inventor of the Sleeping Beauty 100x system) and Prof. Dr. Wolfgang Uckert (expert in T cell therapies). Long standing expertise in the field of cell and gene therapies drive the development of MDCell.
Helmholtz Innovation Lab
The MDCell is supported by a Helmholtz Innovation Lab grant.
Further details about the Helmholtz Innovation Labs initiative
Header image: T-cells (highlighted red) attacking a cancer cell
Rita Elena Serda, National Institutes of Health/CC By-NC-2.0
Technologies
This is a DNA/RNA-based gene engineering tool that does not require the generation of viral vectors. Thus, it provides a great opportunity to facilitate cell engineering for research and clinical use.
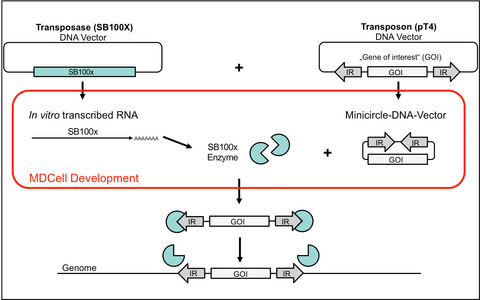
The SB system consists of two components: the transposase enzyme SB100x and the transposon vector pT4. For genomic integration, the SB100x binds to inverted repeats (IR) of pT4 that flank the transgene cassette and paste the transgene into the genome of a host cell. This process is random and SB100x has no preference to integrate in transcriptionally active or oncogenic gene regions.
The MDCell team and the group of Prof. Dr. Wolfgang Uckert was able to improve the gene-transfer system especially fir transfer into primary human T cells by using in vitro transcribed RNA (IVT-RNA) for the transfer of SB100x and a minicircle DNA format for the transfer of pT4 (see Figure 1).
Figure 1: Improved SB100x / pT4 gene transfer system
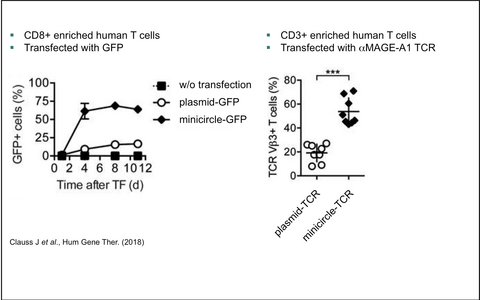
MDCell and the group of Prof. Dr. Wolfgang Uckert applied the improved SB100x/pT4 gene transfer system in primary human T cells. This method enables stable transfection rates of TCRs or CARs of 50% upon a single modification step while maintaining a high survival rate and full functionality of the T cells (see Figure 2).
Figure 2 - Improved SB100x / pT4 gene transfer into primary human T cells
References
Ivics Z, Hackett PB, Plasterk RH, Izsvak Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 1997;91:501-10.
Mates L, Chuah MK, Belay E, et al. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nature Genetics 2009;41:753–61.
Wang Y, Pryputniewicz-Dobrinska D, Nagy EE, et al. Regulated complex assembly safeguards the fidelity of Sleeping Beauty transposition. Nucleic Acids Research 2017;45:311–26.
Clauss J, Obenaus M, Miskey C, et al. Efficient Non-Viral T-Cell Engineering by Sleeping Beauty Minicircles Diminishing DNA Toxicity and miRNAs Silencing the Endogenous T-Cell Receptors. Hum Gene Ther 2018;29:569-84.
Services
MDCell offers its cutting-edge technologies as tailored service to enable customers efficient gene engineering of cells, high-level protein expression as well as pre-clinical assays for gene and cell therapy development with specific expertise around primary human T cells.
- Optimization of TCR or CAR sequences
- Cloning of genes into viral and non-viral vectors (esp. SB100x system)
- Stable viral and non-viral (esp. SB100x-mediated) gene transfer into primary cells and cell lines
- Functional in vitro testing of modified T cells: receptor expression, cytokine expression, functional avidity, cytotoxicity, proliferation
- Functional in vivo models: s.c. and i.v. transfer of cancer cells, treatment with stable TCR/CAR-modified T cells
- Safety: copy number analysis, integration site analysis
- Production of therapeutic proteins
Partnering
Need a solution for your cell engineering project?
MDCell provides physical lab infrastructure and scientific expertise around technologies, where patent rights are held by the MDC.
The concept of the MDCell is to operate financially independent. Project expenses need to be covered by customers and industry partners. MDCell is located under the umbrella of the MDC having access to world-class infrastructure and core facilities at the MDC.
Send us an informal quote of your needs!
Dr. Jan Pille
jan.pille@mdc-berlin.de
Phone: +49-(0)30-9406 3536
The MDCell is supported by experts from the technology transfer office of the MDC and by the qualified team of technology transfer partner Ascenion (contact Michael Karle: karle@ascenion.de).
- PlasmidFactory
-
PlasmidFactory produces plasmids and minicircles according to client’s individual requirements.
-
PlasmidFactory GmbH & Co. KG is a biopharmaceutical company founded in 2000 in Bielefeld, Germany. It has since developed into an international company. PlasmidFactory produces plasmids and minicircles according to client’s individual requirements in modern laboratories with highest quality. In addition to custom manufacturing of plasmid and minicircle DNA, PlasmidFactory focuses its research and development efforts on its core competencies in the production, analysis, application, and storage of DNA.
Cooperation with MDCell
PlasmidFactory´s minicircle technology, resulting in a pure, supercoiled monomeric minicircle, provides the basis for an optimized Sleeping Beauty transposon system, using minicircle mT4 vector and SB100X transposase-encoding RNA, that facilitates the rapid non-viral generation of engineered T cells for immunotherapy.
- Navan Technologies
-
NAVAN developed a technology called Nano-Straws to deliver materials into hard-to-transfect-cells in a non-perturbative manner.
-
In 2017, NAVAN Technologies was founded by a team of nanoengineers, cellular/molecular biologists, and entrepreneurs from Stanford University. Over several years, the NAVAN team developed a versatile technology called Nano-Straws which establish a direct, physical connection into cells to overcome a huge challenge faced by researchers globally: delivering materials into hard-to-transfect-cells in a non-perturbative manner. After extensive in-house testing across a multitude of cell lines and cargos, NAVAN developed an instrument to streamline the delivery of precious cargos directly into sensitive cells which would easily integrate into standard laboratory workflows. A number of national and international academic research labs were engaged to test the enabling potential of Nano-Straw technology in real laboratory conditions. The results validated the instrument’s value and testers confirmed controlled deliveries were successfully completed, without compromising cell viability or function. NAVAN is now focused on commercializing their Nano-Straw technology to enable next generation research and therapies with key-opinion-leaders all over the world.
The MDCell Helmholtz Innovation Lab and NAVAN Technologies, Inc. are actively collaborating with the shared mission of combining the virus-free, reliable and safe Sleeping Beauty transposon system with the fast, effective and gentle intracellular delivery technique of Nano-Straws to reprogram human T-cells. The purpose of this collaboration is to accelerate the development of T-cell immunotherapies for the treatment of cancers and other incurable diseases. - Formula Pharmaceuticals
-
Allogeneic, non-viral CAR therapy platform, leveraging Cytokine Induced Killer (CIK) cells.
-
- Captain T Cell
-
Captain T Cell is a pre-seed stage spin-off project from the MDC that develops next generation T cell-based immunotherapies for late-stage cancer patients.
-
For this, the patient’s most potent immune cells - the T cells – are genetically engineered with a receptor (TCR) enabling them to detect and eradicate cancer cells. Captain T Cell has developed a unique TCR and epitope discovery platform that is the only available technology that generates TCRs for immunodominant cancer targets and thus, more effective TCRs. Captain T Cell is currently developing lead candidate TCRs for clinical application.

Captain T Cell won the world’s largest life science business plan competition OneStart (London, 2016) and the jury award for best spin-off idea at the BioVaria conference (Munich, 2017).
Contact the project leader of Captain T Cell for further information:
News
Key publications
- Mates L, Chuah MK, Belay E, et al. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nature Genetics 2009;41:753–61.
- Wang Y, Pryputniewicz-Dobrinska D, Nagy EE, et al. Regulated complex assembly safeguards the fidelity of Sleeping Beauty transposition. Nucleic Acids Research 2017;45:311–26.
- Clauss J, Obenaus M, Miskey C, et al. Efficient Non-Viral T-Cell Engineering by Sleeping Beauty Minicircles Diminishing DNA Toxicity and miRNAs Silencing the Endogenous T-Cell Receptors. Hum Gene Ther 2018;29:569-84.
- Ivics Z, Hackett PB, Plasterk RH, Izsvak Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 1997;91:501-10.