Studying the human brain in the lab
What’s fascinating about organoids is that they are complex human model systems.
Working with organoids promises to give researchers an effective way to simulate human development and disease in 3D. In autumn 2019, the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC) established a new technology platform focused on using lab-cultivated mini-organs to model disease processes and gain deeper insights into their spatial interactions. MDC researchers will first investigate neuronal diseases before studying other disease types. The platform, which builds on the MDC’s extensive expertise in stem cell and genome editing technologies, is based at the Center’s Berlin-Mitte location and will collaborate closely with Professor Nikolaus Rajewsky’s lab as well as other research groups at the Berlin Institute for Medical Systems Biology (BIMSB).
“What’s fascinating about organoids is that they are complex human model systems,” says Dr. Agnieszka Rybak-Wolf, the head of the new platform. “Organoids therefore bridge the gap between in vitro cell cultures and living animal models.”
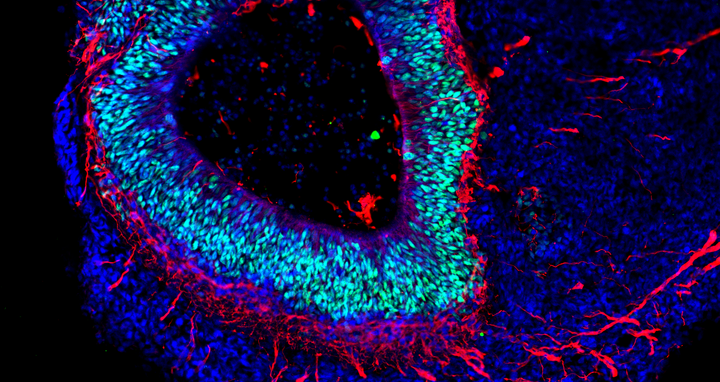
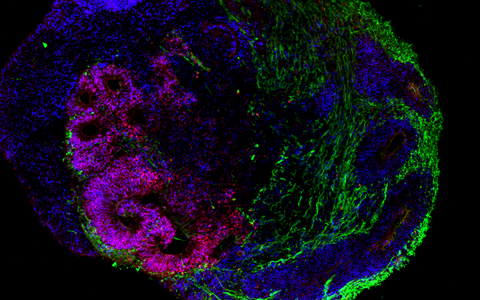
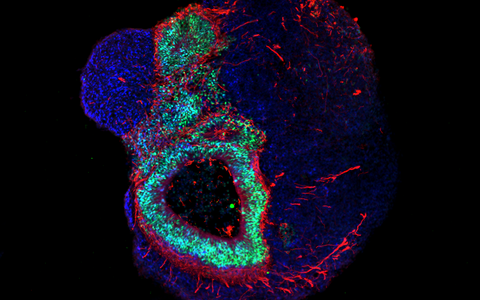
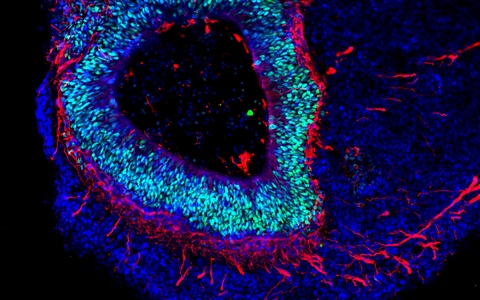
The organoid technology has been getting a lot of attention from numerous research groups around the world because of the potential it has. Its basic principle is that organ-like structures are grown in the lab from human stem and progenitor cells. Molecular signal substances regulate cell differentiation in various cell types, and special culture systems promote an often amazingly realistic 3D self-organization. “Today, brain organoids are typically just a few millimeters in size and reach a stage of development close to that of human organs 23–28 weeks after fertilization,” says Rybak-Wolf, adding that “it is nevertheless possible to use the 3D cultures to mimic the cellular composition and many functional properties of a wide range of tissue types, including parts of the brain, lungs, liver and intestines.”
The technology will be constantly improved
We hope to come extremely close to real organs through the 3D structure of the organoids.
“We hope to come extremely close to real organs through the 3D structure of the organoids,” says Dr. Sebastian Diecke, who has helped establish organoid research techniques at the MDC’s Pluripotent Stem Cells Platform, which he also heads. The teams led by Diecke and Rybak-Wolf, whose methods complement one another and in some cases overlap, plan to work closely together and jointly serve as the contact point for organoid research at the MDC.
Rybak-Wolf’s research group focuses on studying how the transcriptome is altered in the development of healthy and diseased organoids, in other words, how genetic information is transferred from DNA to RNA. To achieve this, it uses state-of-the-art, high-resolution sequencing methods such as single-cell RNA sequencing, known as scRNA-seq. The approach promises to provide a better understanding of the cellular interactions that occur in development and disease processes. Her team also wants to further develop the organoid technology so that complex and artificially vascularized brain organoids can mature in the lab over long periods of time.
The platform led by Diecke, in contrast, concentrates on investigating electrical signaling in brain organoids and on making the novel 3D cultures more stable and reproducible. It is also building a library of pluripotent stem cell lines with defined properties for a wide range of research projects. The team obtains the stem cells by reprogramming human cells into induced pluripotent stem (iPS) cells. Since many diseases can only be studied using genetically modified stem cell lines that were genomically edited with techniques such as CRISPR-Cas, the organoid projects will also draw on the expertise of the MDC’s Transgenics Platform, which is headed by Dr. Ralf Kuhn. Cross-platform projects and applications for third-party funding are possible as well.
On the road to precision medicine
Rybak-Wolf’s platform is already using mini-brains to investigate diseases such as Leigh syndrome – an early-onset, usually fatal genetic disorder of the brain that animal tests can’t be used to study. This research is being carried out in collaboration with Professor Alessandro Prigione’s lab. Working together with the neuropathologist Professor Frank Heppner from Charité – Universitätsmedizin Berlin, the platform is instead using brain organoids to detect as early as possible the changes that occur in brain cells during the development of Alzheimer’s disease. And together with Professor Markus Landthaler, the platform investigates how viral infections influence embryonic brain development. Rybak-Wolf also plans to use organoids to model brain tumor invasion in order to better understand the interplay between healthy and diseased tissues. In addition, various other research groups at the MDC are using organoid models to study topics such as the development of kidneys (Professor Kai Schmidt-Ott) or the neuromuscular system (Dr. Mina Gouti).
The Organoid Platform plays an important role in the EU-wide research initiative LifeTime, which is jointly coordinated by Nikolaus Rajewsky, Director of the Berlin Institute for Medical Systems Biology, and Dr. Geneviève Almouzni from the Institut Curie in Paris. LifeTime aims to analyze the temporal and spatial interactions between human cells and how they change during disease processes – the three-dimensional organoid cultures are a key technology for this research.
In the long term, organoids could supplement or even partially replace animal testing. Researchers hope that human 3D cultures will allow them to study many effects of genetic mutations as well as molecular drug effects more realistically than with animal model organisms. One prospect here is personalized drug tests. As pilot studies have shown, researchers can produce disease-specific organoids from patients’ cells and then use them to test a drug’s effectiveness. Such research takes us a step closer to individualized precision medicine.
Text: Martin Lindner