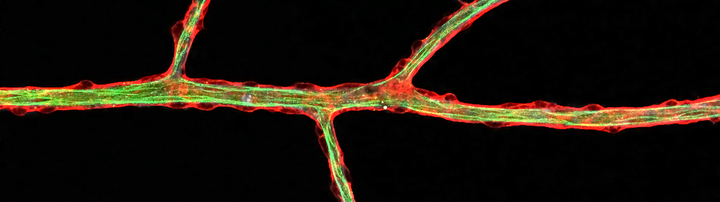
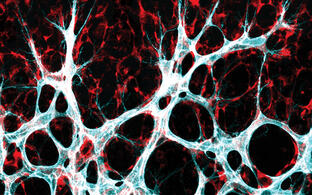
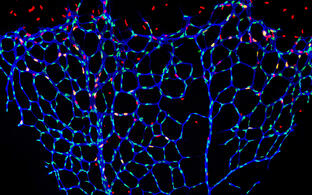
Growth and remodeling of the vasculature are intimately linked to the metabolism of the tissue it supplieswhether this tissue is a developing organ or a pathological anomaly like a metastasizing tumor. Research in the Potente laboratory aims to uncover mechanisms underlying this metabolic linkage.
We study how vascular cells sense metabolic signals and how they use this information to build networks of organ-specific size, shape and differentiation. We are also interested in the metabolism of these cells. Defining the nutrients that they use and the metabolites they produce may reveal mechanisms through which altered metabolism influences vascular function in development and disease. Our goal is to identify regulatory principles whose disruption drives vascular pathology.
We use various genetic, biochemical and imaging technologies, which allow us to define the role of single metabolites, genes, and cells in vitro and in vivo with high spatio-temporal resolution. In the past, this has led to important scientific discoveries at the interface of developmental biology, metabolism and cell signaling. For instance, we revealed that nutrient-sensitive protein modifications control the signaling dynamics of essential angiogenic pathways such as Notch and that changes in the levels of these modifications adjust vascular patterning. We also identified molecular determinants of vascular metabolic activity, including the transcriptional regulators YAP/TAZ and MYC, which prepare endothelial cells for growth and proliferation. Moreover, we uncovered essential metabolic pathways limiting blood vessel expansion and described the forkhead transcription factor FOXO1 as a central coordinator of these programs.
Overall, our laboratory emphasizes collaboration and prioritizes approaches that can inform about fundamental biological principles while also pointing the way towards clinical insight.
For further information, please visit our lab webpage (www.angio-met-lab.com).