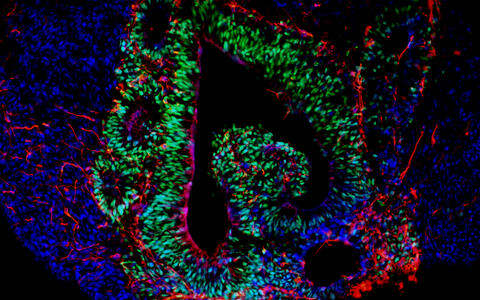
Mini-organ, mathematical model or mouse?
Biomedicine ceased to rely solely on animal experiments a long time ago. Now, mini-organs are grown in petri dishes, algorithms sift through vast amounts of sequencing data and can even make predictions, and organ replicas are stored on chips. But does that mean we can do away with animal research entirely? Professor Jana Wolf is not optimistic: “It took more than 200 years to get from Newton’s description of the laws of gravity to the first flight simulators, and many experimental tests were necessary along the way. In biology, we haven’t even deciphered all the basic laws yet.”
In biology, we haven’t even deciphered all the basic laws yet.
The biophysicist develops models for both normal cellular processes and disease states. The quality of her models is dependent on the basic assumptions being correct. And even then, any calculations the computer makes about living nature must be subsequently verified by others in experiments. Often, this is only possible with animal experiments. So, in December 2020, Wolf – together with some 40 other scientists at the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC) – took a stand and explained why, in her view, non-animal methods are not an alternative but a complement to in vivo research that complete the picture.
As a general rule, researchers planning experiments with mice, rats or other animals must first prove that it is the only viable method. “It is a legal requirement that all other options have been ruled out,” explains Nadja Daberkow-Nitsche, an animal welfare officer at the MDC and head of its animal facilities. The number of animal experiments should also be kept to a minimum, and involve as little distress or suffering to the animals as possible.
Roughly one-third of research labs at the MDC work entirely without animal experiments, like Jana Wolf. But the majority of the more than 70 labs use both non-animal methods and animal experiments to help them understand the processes that take place in states of human health and disease.
- What does in vivo, in vitro, in silico mean?
-
Organism, petri dish and computer
-
In vivo refers to when scientists study processes within a living organism, while in vitro methods are used to replicate natural processes in an artificial environment – for example, in a test tube or petri dish.
Researchers can also model and study physiological processes in silico, i.e., on the computer. Such mathematical models are based on data from laboratory experiments.
Cultivating cells outside of an organism
Cell cultures are standard in science. Within the artificial environment of the petri dish or test tube, the situation is less complex than it is in vivo. The study conditions can be better controlled and reproduced in follow-up experiments.
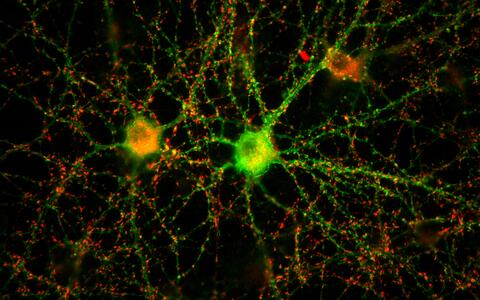
A neuronal network of two nerve cells in the petri dish.
The cells closest to those found in the living body are primary cells, which come directly from the blood or tissue of donors and patients. However, primary cells age and die quickly, and can only be kept in cultures for a few days. Furthermore, biopsies are often difficult or impossible to obtain – for example, in the case of lung or brain biopsies.
Scientists around the world also work with immortal cell lines that originate in tumor cells or have been modified in the laboratory so they can divide indefinitely. However, the transformation into a state of immortality costs the cell some of its original properties: such permanent cultures have an altered cell metabolism and are sometimes no longer able to attach to one other; heart cells no longer beat; and cell types look completely different from those in the cell structure of the original organ.
- Primary cell cultures: Examples from the lab
-
Closest to the cells in the living body
-
Primary cultures can reveal a lot about how diseases or allergies develop. The MDC lab of Professor Young-Ae Lee is stimulating primary immune cells from the blood of allergic children. She wants to learn how these cells respond to such stimulation. Her team is also using single-cell analysis to characterize the different subtypes of immune cells obtained from the patient samples, which promises to shed more light on the immune response as a whole. Many mathematical models also rely on sequencing data derived from primary cultures.
- Transformed cells: Functional studies with HeLa
-
Immortal cell lines
-
Scientists worldwide are working with immortal cell lines. The robust cells are suited for use in functional studies, such as investigations of the cell cycle or cellular metabolism. They can also be genetically modified much more easily than primary cells or even living organisms. What’s more, such cells are inexpensive and readily available. Researchers can simply order them over the internet from a cell database. “We are studying fundamental molecular processes, looking in particular at yeast cells, some of whose processes are very similar to those in human cells. To conduct such studies, we primarily need a large number of cells that can be easily manipulated. The cell type itself is not that important,” says Dr. Emanuel Wyler, whose research at the MDC’s Berlin Institute for Medical Systems Biology (BIMSB) involves using HeLA cells to investigate gene regulation.
The abbreviation HeLa stands for Henrietta Lacks, an American woman who contracted an aggressive cervical carcinoma in the 1950s. Her doctors cultivated the first human immortal cell line from a sample containing her tumor cells. To this day, HeLa cells are used all over the world in medical and basic research.
Stem cells from the lab
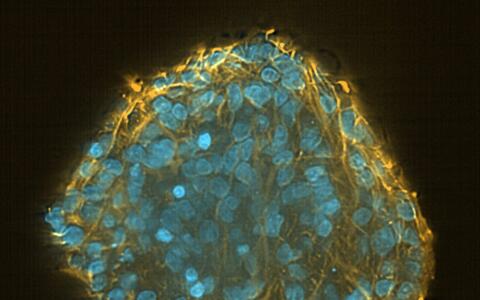
It is for this reason that many researchers are turning to induced pluripotent stem (iPS) cells. These all-rounders look quite unspectacular, almost like ordinary cells. With a large cell nucleus surrounded by cytoplasm, they crowd closely together to form small islands on the base of the petri dish. If you were to take a look through one of the microscopes set up in the laboratory of Dr. Sebastian Diecke, head of the MDC’s Pluripotent Stem Cells Platform, you would hardly guess the talents of the iPS cells.
Induced pluripotent stem cells in a pluripotency test
Theoretically, iPS cells have the potential to become any cell type in the human body. Unlike primary stem cells, taken from muscle for example, they do not die in the cell culture, and unlike embryonic stem cells, they are not surrounded by ethical controversies. “Many people hope they will eventually be able to replace animal testing,” says Diecke.
All his team needs are simple cells taken from the skin or blood to produce these stem cells. Using a cocktail of four genetic factors, they reprogram these normal cells into pluripotent stem cells, which they can then use to develop a tissue-specific cell type – for example, a neuron or a heart muscle cell.
Stem cells in cardiovascular research
Professor Norbert Hübner’s lab works with such artificially generated heart muscle cells. They have similar properties to real cells and, in line with the 3R principles (Replace, Reduce, Refine), are not isolated from mouse or rat hearts. “However, they do not reach the level they would have reached in the adult heart – remaining immature and in a near-fetal state,” says Dr. Henrike Maatz. Cells that are cultivated for months in an incubator are also very time-consuming and expensive: “We need to feed them every day.”
Patient-specific iPS cells have the same genetic makeup as their donors. They map the disease at the molecular level and are therefore often used for initial drug tests. However, preliminary experiments are necessary first.
Stem cells in Alzheimers's research
Alzheimer’s researcher Professor Thomas Willnow, for example, used a cell culture to first investigate what the ApoE4 gene variant, a protein, does at the molecular level in the nerves, and why it significantly increases the risk of dementia. This is how he discovered a disease-causing process – a defect in the fat metabolism of the neurons. His team had to then verify these results in mouse models in order to rule out any possible interactions with metabolic processes in the liver or intestine in the living organism. Only then could they go forward with further investigations using human neurons from iPS cultures. This way, his team now wants to find substances that help get the disrupted metabolic process in the cell back on track.
Following drug tests in cell cultures, scientists must confirm the efficacy and safety of the substance – which again requires animal experiments. Only then can clinical trials with humans begin. “We can see in vitro whether a potential therapeutic agent against Alzheimer’s alters the progress of the disease in individual cells or cell assemblies – but the influence on memory performance only becomes apparent in behavioral studies with animals,” Willnow explains.

Prof. Thomas Willnow and Dr. Anna Löwa talk about the causes of Alzheimer's disease and experiments with mice and brain organoids.
Stem cells in immunology
In vitro methods also reach their limits in biomedicine when there are several possible factors that increase the risk of a disease. Dr. Alexander Mildner, for example, is researching the autoimmune disease multiple sclerosis (MS), which can develop in different ways in each patient. He wants to know how immune cells manage to cross the blood-brain barrier in MS patients and cause damage to the central nervous system.
This barrier normally protects the central nervous system from cells that should not be there. “Both the blood-brain barrier and the nervous system are composed of a multitude of different cell types that are in constant exchange and only work in interaction with each other,” says Mildner. To date, it has been technically impossible to reconstitute such cell assemblies into complex three-dimensional tissues in vitro. Moreover, not all human body cell types can yet be produced from iPS cells. In the case of immune cells, for example, the process of differentiation that occurs during the course of a disease has not yet been researched well enough.
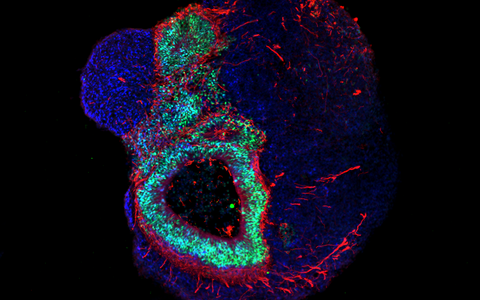
Organoids – „mini-organs“ from the petri dish
Organoids mirror the interactions of multiple cell types. These mini-organs originate from iPS cells that, when triggered by artificially generated signals, spontaneously organize themselves into three-dimensional organ-like structures consisting of several different cell types.
They may be only a few millimeters in size, but they bring biomedicine a big step forward. “No other in vitro cell culture model provides such a level of cellular complexity and so well reflects human organs composition and function,” says Dr. Agnieszka Rybak-Wolf, head of the Organoids Platform at the MDC. Diecke and Rybak-Wolf mainly create brain organoids, but organs such as the lungs, liver or intestines can also be mimicked in this way. A team led by Dr. Mina Gouti even managed to get a neuromuscular organoid made of neurons and muscle cells to contract.
- Assembloids
-
Fusing multiple brain organoids
-
Assembloids are created by fusing several brain region specific organoids. They form neural circuits that link cells of different brain regions. The interaction between neurons, progenitor cells, oligodendrocytes and astrocytes could provide clues to what happens differently in the brains of sick people – albeit during the embryonic developmental stage.
Dr. Silke Frahm-Barske from Diecke’s team sees organoids as a complement to animal experiments. She observed in mice that stress has an influence on a protein that is found in excess in Parkinson’s patients, and was subsequently able to demonstrate the same effect with organoids that originated from cells of a patient. This demonstrated that her assumption that stress plays a major role in Parkinson’s is transferable to humans.
Even using a chemical from a different manufacturer can have a big impact.
Nevertheless, Professor Erich Wanker, whose research topics includes Huntington’s disease, a hereditary neurological disorder, currently prefers to use a different model for genetic studies: “In flies, we can very precisely intervene in the genome and the results are more reproducible,” he says. With organoids, he explains, no one is ever the same as another. This is partly due to different genetic conditions in the starting material – the patients’ cells. In addition, there are many factors at play in the production of organoids that are either uncontrollable or very susceptible to the slightest changes. “Even using a chemical from a different manufacturer can have a big impact,” says Wanker.
So far, it has also not been possible to create organoids with their own blood circulation. The organ-like structures are therefore dependent on nutrients supplied externally by researchers. If they get too big, part of the organoid does not receive enough of these nutrients, start to form a necrotic core and dies.
- A new Einstein Center in Berlin
-
Berlin network for the development of alternative methods to animal experiments in biomedical research
-
In 2021, the MDC and other Berlin research institutions are teaming up to establish the “Einstein Center 3R.” The network’s aims include developing better 3D tissue models, improving the translation of laboratory discoveries to patients, and strengthening animal welfare. In the planned center, young scientists will be able to rigorously apply the 3R principles through proper training, instruction and continuing education. MDC researchers want to use single-cell analysis to advance techniques involving organoids and stem cell technologies. The MDC will also collaborate on efforts to model diseases such as Alzheimer’s, Leigh syndrome, glioblastomas and infectious diseases. The goal is to develop personalized gene therapies for patients.
Numerically modeling reality
Not all molecular biologists experiment in the laboratory with pipettes, microscopes and other equipment – some are busy programming, feeding data into models, or developing algorithms. It is now possible to virtually simulate processes in the living body and thus map cellular signaling pathways in healthy and sick people, learning at what point in time which genes switched on, and when gene regulation gets out of control. These models are built on biological data such as sequencing data and can show, for example, how microorganisms in the gut interact with the cells of the human host to bring about diseases.
In the Akalin lab scientists analyse big data sets.
Such unknown influences can never all be incorporated into one model.
Dr. Sofia Forslund compares such models to a crystal ball, where gazing into it reveals the direction where research should look first. Thousands of factors can be involved in inflammation in gut and body, for example, but a data model limits the selection of possible drivers quite considerably. “This allows us to narrow down potential hypotheses,” she says. Yet in real life, environmental factors also affect the host-microbiome interactions, such as the subject’s social contacts or diet. “Such unknown influences can never all be incorporated into one model,” says Forslund.
Prior assumptions made by the researchers form the basic framework of every mathematical model. “We always start with a hypothesis, and we then have to decide which processes are important and which are not for the problem we are looking at,” explains Professor Martin Falcke, who models physiological processes in the life sciences. “Animal experiments are indispensable for testing whether our initial assumptions were correct.”
Alternatives to animal experiments as complementary methods
Every research model is only ever an image or a possibility of reality – a simplification of the natural situation. This applies to simple cell cultures as well as to induced stem cells – the touted all-rounders of the cell lines. Neither the more complex organoids nor computer simulations can yet completely replace animal experiments in research. Even animal testing itself is only an approximation.
As a result, most researchers at the MDC do not speak of “alternatives” to animal experiments, but of “complementary methods.” For them, one thing is certain: in vitro and in silico studies can help adhere to the 3Rs and reduce the number of animal experiments, but they do not yet represent a substitute.
Text: Christina Anders