Siffrin Lab
Neuroimmunology Laboratory
Profile
Disability in MS is directly related to the degree of axonal damage. It has been shown that the induction of neuroaxonal damage is present early in the disease process and phases of damage and repair take place continuously.
Our main goal is to understand the complex dynamic interactions of immune cells with CNS cells. Using various techniques, we visualize CNS and immune cells and cellular effector functions, e.g. Ca2+ dynamics or cytokine expression in animal models and in human cell culture models. This serves to understand the sequential steps of immune cell penetration and local tissue damage processes. These studies are accompanied by standard cellular and molecular biological analyses.
Our work aims to identify new therapeutic targets that can be used to prevent or regenerate neuroaxonal damage.
Team
Group Leader
PD Dr. med.Volker Siffrin
Scientists
Dr. Marlen Alisch
Dr. Jessy Chen (Jun.-Clinician Scientist / BIH)
PhD Students
Charlotte Biese
CharlotteGillaLouise.Biese@mdc-berlin.de
Bakhrom Muinjonov
bakhrom.muinjonov@mdc-berlin.de
Students (Bachelor/Master/Medical)
Luise Reukauf (Cand. med.)
Gesche Herold (Cand. med.)
Franziska Försterling
Tess Kühl
Technical Assistants
Jana Engelmann
Andrea Behm
ALUMNI
Kamil Sebastian Rosiewicz
Janis Kerkering
Dr. med. Lil Antonia Meyer-Arndt
Tadhg Crowley
Research
Project: MS and EBV - How can viral infection trigger CNS autoimmunity? (DFG-funded)
A severe first infection with the human B-cell-transforming Epstein-Barr virus (EBV) - infectious mononucleosis - represents a risk factor for developing MS later in life. In the course of evolution, the EB virus has developed mechanisms to instrumentalize the human immune system in order to establish optimal co-existence with its human host. This leads to high viral persistence and prevalence of EBV in the human population throughout the world. At the same time, the human immune system has developed efficient mechanisms of adaptive immunity, which are based in particular on the generation of efficient memory T cells to keep the virus under control. A potential causative role for the triggering of autoimmune diseases by the EBV-driven immune reaction has been discussed for some time, but has not yet been proven. We study how viral proteins alter B-T cell interaction to result in CNS autoimmunity. These investigations are of great clinical importance, since the results could indicate a more specific treatment option for MS. B-cell depletion is currently one of the effective treatment options for MS, which has the disadvantage of strong immunosuppression. Our work has the potential to show a more specific treatment of B cell function in the disease.
Project: MS and astrocytes - How can astrocytes modulate neuroaxonal damage in CNS autoimmunity?
Astrocytes are involved in MS early on, perhaps even before oligodendroglial damage, and have multiple potential roles in the process of damage and repair in MS lesions. Astrocytes as part of the blood brain barrier clearly interact with invading immune cells, they present antigen to T lymphocytes by MHC molecules in animal models of MS. In the CNS white matter astrocytes are of utmost importance for the energy supply of the axono-oligodendroglial unit. If astrocytes are depleted in the animal model of MS, an increase of immune cell infiltrates and exacerbation of clinical signs has been observed. On the other hand, it has been shown that astrocytes were able to critically entice inflammatory CNS processes and neuronal damage formation by autocrine glycolipid-mediated astrocyte activation. Therefore astrocyte modulation is a potential lever to treat inflammatory neuroinflammation. We analyze astrocytes in animal models as well as in human culture models to identify therapeutic targets to improve neuroaxonal damage formation in MS.
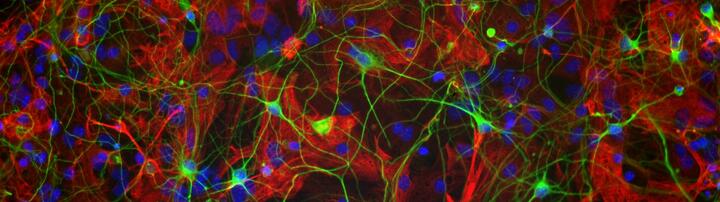
Project: MS in a dish - Modeling aspects of the disease in a human context
Tackling neurodegeneration is one of the major common unresolved problems in neurology not only in the classical neurodegenerative disorders such as Alzheimer’s disease or Parkinson’s disease but also throughout disease entities such as vascular (stroke), MS (autoimmune inflammatory) and traumatic brain diseases. In this context, there is increasing evidence, that e.g. human astrocytes and microglia have a more complex scope of functions than rodent ones. This might be one of the reasons that in translational approaches based on results in animal models up to now no clear strategy could be identified to specifically address the neurodegenerative aspects of these diseases. Therefore, the need of in vitro models with human/patient-derived CNS cells is evident. We have established protocols for the differentiation of human iPSC/NSC to neurons and astrocytes as well as microglia differentiation from iPSC/HSC. We use these 2D-models in co-cultures of diverse CNS derived cells for the analysis of our research questions. We currently establish 3D models by transplanting these human glial cells into mouse brain slices for in vitro organotypic cultures. In parallel, we are working on brain organoid differentiation in cooperation with the stem cell facility of the MDC. Structured and more complex CNS structures are relevant to create representative in vitro models of MS.
Publications
News
Jobs
There are no positions open at the moment.
However, we encourage medical students interested in a medical doctor thesis or natural science students interested in master thesis work to apply at any time.


