Lewin Lab
Molecular Physiology of Somatic Sensation
Profile
Somatic sensation includes all those sensations that we consciously feel after stimulation of the body, e.g. fine touch, warmth, cooling, limb movement as well as unpleasant sensations like burning and blunt mechanical pain. We experience these sensations as a direct result of the activation of sensory neurons that are located in the dorsal root ganglia (DRG). We aim to discover the molecules involved transforming mechanical and thermal stimuli into electrical signals that lead to perception (see Paricio-Montesinos et al. Neuron 2020 reprint; Schwaller et al. Nat Neurosci 2020 reprint). We are active in identifying proteins and mechanosensitive channels required for touch sensation. An emerging theme from our lab is also that mechanotransduction proteins have roles in other non-sensory systems e.g. cancer metastasis (Patkunarajah et al. eLife 2020 reprint) and joint growth and maintenance (Servin-Vences et al. eLife 2017 reprint).
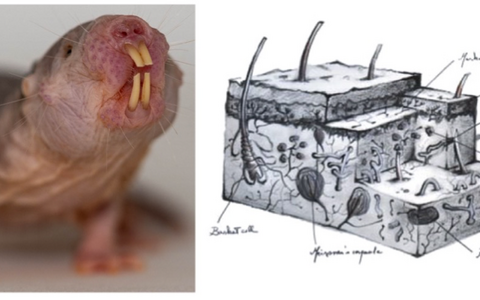
Left, a naked mole-rat posing for us in the Lewin lab. Right an artist’s impression of the complex arrays of sensory receptors present in the skin of humans - a picture that is very similar in almost all animals on earth.
Somatosensory research led us to start studying the naked mole-rat more than 15 years ago, as together with Thomas Park we showed that these animals lack certain types of pain sensations (see Park et al. PlosBiology 2008 reprint). Subsequently, we started working on multiple aspects of naked mole rat’s extreme biology. In the last 10 years we have shown how mole-rats are impervious to acid pain (Smith et al. Science 2011 reprint). How mole-rats can survive unscathed complete oxygen deprivation by using fructose as a fuel (Park, Reznick et al. Science 2017 reprint). We have also expanded our research to related African mole-rats, some of whom also display “superpowers” like complete imperviousness to an active ingredient of mustard gas (Eigenbrod et al. Science 2019 reprint). Most recently we have started to explore the neurobiological basis of the social nature of the naked mole-rat including the discovery that these animals have a vocal culture (Barker et al. Science 2021 reprint).
Team
Group photos
Research
Sensory neurons of the dorsal root ganglia allow us to detect stimuli to the body surface that lead directly to the sensations such as touch and pain. In my group we are interested in the genes that allow these neurons to transduce different types of stimuli. Sensory neurons can, for example, detect changes in temperature of the skin in non-noxious (not painful) as well as the noxious range (painful heat or cold). They can also detect gentle movement of the skin as well as intense mechanical stimulation of the skin that is normally harmful. The nature of the transduction molecules involved together with the developmental events that lead to specification of the appropriate sensory neuron sub-types are actively investigated in my lab.
Research Projects
- 1. Molecular Physiology of Somatic Sensation
-
Research project 1
-
Somatic sensation includes all those sensations that we consciously feel after stimulation of the body, e.g. touch, warmth, cooling, or even limb movement. We experience these sensations as a direct result of the activation of sensory neurons that are located in the dorsal root ganglia (DRG)....
- 2. Neuronal nanodetection and micro-patterning, engineering sensory neurons for function
-
Research project 2
-
The mechanosensitive ion channels that are expressed by sensory can be measured using high-resolution electrophysiology techniques. We have recently shown that such ion channels in the membranes of cultured DRG neurons can be activated by stimuli in the nanometer range....
- 3. Touch, hearing and the development of mechanosensation
-
Research project 3
-
Hereditary deafness is a relatively common phenomenon and a large number of genes have been identified that when mutated lead to deafness in mouse and man....
- 4. Tuning pain sensitivity
-
Research project 4
-
Nociception is describes our ability to respond to potentially or actually damaging stimuli. An important aspect of the biology of nociception is that after injury people and animals become much more sensitive to sensory stimulation than before injury....
- 5. The Naked Mole Rat a pain free mammal?
-
Research project 5
-
The naked mole rat is an unusual subterranean rodent in many respects. It is the only known poikilothermic mammal (ie. cold blooded), it lives in colonies with an insect-like social structure, and it is also the longest-lived rodent species known (lifetimes in excess of 25 yrs)....
Methods
- Electrophysiology
-
-
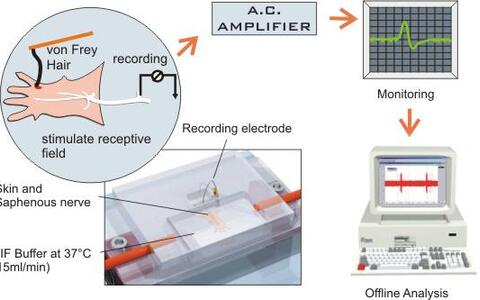
Five electrophysiology rigs are set up in the laboratory. Two setups are devoted primarily to extracellular recording from sensory afferents innervating the skin in vitro. This preparation was originally developed to record from nociceptive afferent fibers in the rat (Reeh, 1986). Martin Koltzenburg and myself (Gary Lewin) developed this preparation for recording from mouse and embryonic chick afferent neurons innervating skin (Airaksinen et al. 1996; Koltzenburg et al. 1997; Koltzenburg and Lewin, 1997). This preparation has the advantage that one can quantitatively measure changes in the physiological properties of functionally defined afferent neurons in animals subjected to a targeted mutation of any given gene.
Fig1. A schematic diagram of the skin nerve preparation
© drawing by Paul HeppenstallWe also currently have two electrophysiological set-ups devoted to whole cell patch clamp recording from acutely-isolated mouse dorsal root ganglion neurons (A new set-up is currently being built). Using this preparation, one can monitor biophysical properties, such as voltage activated currents, and action potential parameters of neurons taken from mice with targeted disruption of specific genes.
Fig2. Patch Clamp set-up
- Molecular Biology
-
-
A variety of standard molecular biological techniques are used in the laboratory. Molecular cloning is used to isolate DNA sequences as templates to make cRNA probes for northern blotting and in situ hybridization experiments. We have used 5' RACE techniques to isolate novel cDNA sequences. In order to directly assess the function of genes in specific biological processes (such as sensory mechanotransduction) we have also generated knockout mouse models.
- Cell Biology
-
-
We routinely use cultured cell lines and primary sensory neurons from chicken, mouse and rat. We have also established cell lines that overexpress epitope tagged versions of mammalian mec gene homologues. We use such methods to express ion channel proteins together with proteins that might modulate the kinetic properties of these channels. Cell lines are examined using patch clamp techniques
Fig3. Cell culture room
Links
The group's latest publication "Hypofunctional TrkA Accounts for the Absence of Pain Sensitization in the African Naked Mole-Rat" (Omerbašić et al., Cell Rep., Oct 2016) has triggered some articles in public media.
Publications
News
Technology transfer
Christiane Wetzel, Gary Lewin
There is an unmet need for more effective analgesics and novel strategies for analgesic drug development. With Stomatin-like protein-3 (STOML3) we have identified a potential molecular target that directly participates in the transduction of noxious and innocuous mechanical stimuli in sensory neurons.
We found that STOML3 deficient mice have many mechanoreceptors and nociceptors which are essentially insensitive to mechanical stimulation. Interestingly, STOML3 deficient mice show reduced symptoms of neuropathic pain and this may be due to the impairment of touch reception in these mice. We have developed novel high throughput assays for screening small molecules that disrupt STOML3 function. For further validation and lead optimization of small molecules we employ a set of experimental paradigms. For example we can test whether candidate compounds block mechanosensitive currents in isolated sensory neurons using the whole-cell patch-clamp technique. We can further test if local application of our compounds to the skin will block mechanosensitivity at the receptive endings of single primary afferents in the skin using the in vitro skin-nerve technique.
Eventually we will perform behavioral tests in order to determine if the application of novel, potentially analgesic compounds leads to a lack of hypersensitivity to touch evoked pain.