Growing mini-organs from stem cells
Over the past year, MDC researchers successfully used stem cells to grow functional muscle tissue and the spinal cord neurons that control muscle movement. During the Long Night of Sciences, visitors will get a chance to see these cells and tissues up close, and learn why they are so important.
Visitors to the Long Night of Sciences on June 15 will have the opportunity to see something that most haven’t, simply because it is so new: spinal cord neurons and skeletal muscle tissue grown together from stem cells.
While growing tissues from stem cells is now relatively widespread, no one else is known to have grown these key components of the neuromuscular system into miniature organ-like structures, called “organoids.” And that has only happened within the past two years at Max Delbrück Center for Molecular Medicine (MDC).
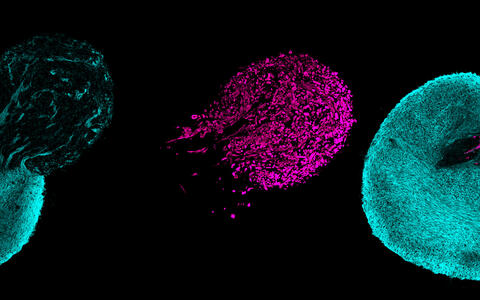
Human stem cells can form three-dimensional, neuromuscular miniorgans. The spinal cord neurons are shown in a blue tone, the skeletal muscle cells are marked purple.
“It really is research that is happening right now,” said Dr. Mina Gouti, who leads MDC’s Stem Cell Modeling of Development and Disease Lab. “For that reason, we are very excited to share it with the public.”
To study and treat disease
The neuromuscular organoids hold great potential for studying development of the neuromuscular system and diseases, such as spinal muscular atrophy and Amyotrophic Lateral Sclerosis (ALS), also known as Lou Gehrig’s Disease.
Movements that we take for granted— walking, drinking coffee, breathing — require muscles to contract, and such contractions are triggered by a message sent by spinal cord neurons. When this communication system breaks down, it can lead to severe conditions.
In the future, Gouti plans to use the organoids to investigate potential treatments, such as helping slow or stop muscle neuron degradation, or even potentially restore communication between the spinal cord and muscles. “This will be a time-consuming, difficult process,” Gouti said. “However, it holds great promise in discovering novel, efficient therapies.”
It starts with stem cells
To grow the organoids, Gouti’s lab works with a particular type of stem cell, called induced pluripotent stem cells (iPSCs). Pluripotent stem cells have the ability to become any type of cell in the body. Molecular biologists can take a tissue sample from a patient, such as skin cells, and reprogram them to revert back into stem cells — these are induced pluripotent stem cells (iPSCs). Then, they try to coax the iPSCs to become the specific type of tissue they want to study. Understanding the cues and other influences that trigger the cells to become say a muscle cell, rather than a brain cell or a heart cell, is key to success.
It was only about a year ago that Gouti and her colleagues succeeded in prompting stem cells to ultimately develop into the spinal cord neurons and skeletal muscle, and growing into a three-dimensional, self-organizing tissue structure in a petri dish.
Patient specific
Importantly, these cells, whatever they become, still carry the mutations of the patient’s DNA, allowing the scientists to more easily study the patient’s disease in the lab. For example, testing how the patient’s cells respond to certain drugs or treatments.
But first the researchers have more basic challenges to address, such as maintaining tissue cells in the lab for an extended time. The organoids can grow for several months, and then begin contracting like muscles do in the body. However, they don’t have a complete blood vessel system to carry nutrients to the center of the mini-organ, so there is a limit to their growth. The researchers also need to understand how the organoid tissue compares to the real thing.
Visitors on the lab tour will get a chance to observe stem cells under the microscope, fluorescent images of neurons and skeletal muscles, as well as the organoids themselves. They will have a chance to see how the research team works to grow and nurture the tissues, and how the organoids develop over time into a functional, contracting muscle.
Text: Laura Petersen