📺 A long and winding road against cancer: from basic research to therapy

A long and winding road – a film with Thomas Blankenstein, Elisa Kieback, Mathias Leisegang, Antonio Pezzutto and Wolfgang Uckert. (with English subtitles)
In the last two years, two drugs have come onto the market that are based on research carried out at the MDC and that use entirely new mechanisms of action: Vonvendi, a form of treatment for a hereditary bleeding disorder, and Blincyto, an immunotherapy against cancer. The MDC is very proud of these success stories. After all, translational research is a cornerstone of its founding mission: Findings from the lab should first and foremost benefit patients.
Though this may sound simple, it is in fact extremely difficult to achieve. Not only does it require a good team, good technology, and good ideas, it also demands a great deal of patience and perseverance. Provided everything goes smoothly, the journey from basic research in the lab to the approval of a new drug takes around 15 years. In our film, we focus on the example of a promising cancer immunotherapy.
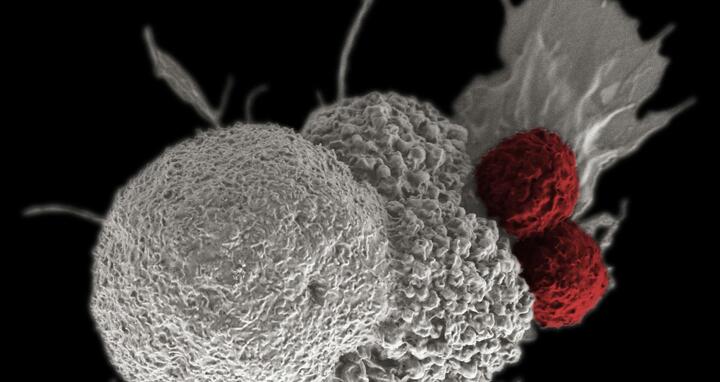
Certain mutations in the cancer cell – sometimes the replacement of just a single letter in its genetic code – can cause the immune system to recognize the cell as “foreign.” Often this mutation simply alters the protein that is displayed on the surface of the cell, which functions much like an ID photo. Manipulating this “photo” allows T-cells – which act as the body’s police force – to recognize the imposter, dock themselves at this particular location, and destroy the tumor cell.
This is how the body usually fights viral infections. The immune system fails when it comes to detecting cancer cells, however, as T-cells no longer recognize the altered photograph. One of the aims of the MDC research groups led by Professor Thomas Blankenstein and Professor Wolfgang Uckert is to train T-cells to detect these photos again. To achieve this, they began by producing human T-cell receptors in a specially designed mouse model. The first patients should start to receive treatment using this therapy in 2018 as part of a phase I clinical trial.
The groundwork for this research began almost 20 years ago. “The first lab entry was recorded in May 2000 by my colleague Liang-Ping Li,” recalls Thomas Blankenstein. “The first publication on this mouse model then came out in 2010.” Liang-Ping Li now works as a professor in Guangzhou, China. But for a long time it was unclear if his trials would ever lead to anything.
“That’s just the way it is with experiments,” says Blankenstein. “It happens to everyone.” He explains that this can be particularly problematic for young researchers who do the actual work in the laboratory. For them, the next stage of their career often depends on the success of these experiments. “It can therefore be very stressful when the findings, and indeed the publications, fail to materialize.” Even for an established group leader, these dry spells are not easy: “I occasionally had doubts about whether it would work,” admits Blankenstein.
This uncertainty and possible failure are all part of daily life in the laboratory – and also one of the topics of our film. And it is certainly not only a feature of translational projects. Anyone conducting research at the frontier of knowledge and uncovering new terrain will encounter unexpected obstacles. At the same time, this research can lead to applications that nobody could have foreseen. The question “What are its uses?” is often only fully answered much later.
There is no shortage of examples where this has been the case. When Michael Faraday demonstrated electromagnetic induction with his experiments, his goal was to understand the universe. At that point, no one could have imagined the extent to which electricity would transform our lives and the enormous benefits it would bring. Similarly, the research into a strange viral defense system in bacteria was initially only of interest to a small group of microbiologists. Today, the Crispr-Cas9 “gene scissors” are an integral tool in many laboratories – including those at the MDC. Such examples demonstrate the creativity of basic research.
“Many experiments […] intrigue generations of researchers, but not the world,” wrote the German philosopher of science Jürgen Mittelstraß. But he stressed that, although such research may at first glance seem like chasing rainbows, it is in fact the very essence of science, that which “constitutes its curiosity and its freedom, and without which it could not exist.” Only pure basic research, Mittelstraß claimed, brings truly new knowledge into the world, rather than that which is familiar or sought after.
The discovery comes first, followed by a new understanding and new questions. And the more fundamental the discovery, the more questions it raises, especially as we get a better sense of all that we do not know – like the circular ripples produced by a stone when it enters the water. Basic research thus inspires curiosity without needing to immediately promise a concrete benefit. It is of value in itself.
At the same time, however, basic research cannot be separated from application. It forms the foundation upon which every translational project is built – including at the MDC, which is now one of the world’s leading biomedical research institutes. A total of 1,660 people currently work at the MDC, studying things like ion channels, T-cell receptors, and the regulation of gene expression in order to better understand the molecular basis of health and disease. This can, at times, be a tedious and lengthy process – for the scientists themselves, for the institution, for the funding bodies, and for society. But one must be patient and persevere.
“For us, good research is also creative and open-ended research,” says Professor Martin Lohse, Scientific Director at the MDC. “Our researchers should be free to pursue discoveries on the horizon – even if the goal is not yet clear and the road to reach it long and winding.”
Author: Kerstin Hoppenhaus
(Kerstin Hoppenhaus is a science journalist and director. She wrote and directed the films for the MDC’s silver jubilee in collaboration with the producer and camerawoman Sibylle Grunze.)
The science behind it
PHASE I CLINICAL TRIAL
Starting in early 2018, patients with multiple myeloma for whom the standard therapy does not work can take part in a phase I clinical trial at Charité’s Medical Department, Division of Hematology and Oncology at Campus Benjamin Franklin. The joint clinical trial of Charité and the MDC is led by Professor Antonio Pezzutto. The patients will be infused with autologous T-cells that are genetically modified with a receptor that targets a specific antigen on tumor cells. The cells will be produced in the GMP laboratory of the ECRC at Campus Berlin Buch. The clinical trial is receiving € 4.1 million from the Federal Ministry of Education and Research as part of its personalized medicine program.
T-KNIFE
T-knife is a spin-off of the MDC that develops T-cell therapeutics for clinical applications. The company has purchased wide-ranging licensing rights from the MDC, including the rights to a technology platform for generating cancer-specific receptors as well as the rights to various receptors and the phase I clinical trial.
CAPTAIN T CELL
In May 2016, a start-up consisting of MDC researchers won the OneStart competition in London with its Captain T Cell project. OneStart is the world’s largest life sciences and healthcare start-up accelerator program. Dr. Felix Lorenz, Dr. Elisa Kieback, Julian Clauß, and Dr. Inan Edes came out ahead against 400 international teams. Their prize money totaled 100,000 British pounds.
HELMHOLTZ INNOVATION LAB
In the spring of 2017, the Max Delbrück Center Cell Engineering Lab (MD-CELL) began its work. This platform for application- oriented research is supported by the Helmholtz Association and relies on close collaboration between science and industry. The MD-CELL has its roots in Professor Wolfgang Uckert’s and Dr. Zsuzsanna Izsvák’s research teams at the MDC. They are developing new techniques that outfit large numbers of immune cells with new genes in a safe and reproducible way. This involves “training” the T–cells to the fight the tumor of a specific patient.
HELMHOLTZ VALIDATION FUND
Among the projects currently funded by the Helmholtz Validation Fund is a project of Dr. Armin Rehm and Dr. Uta Höpken. They are using CAR T-cell therapy to develop a gene therapeutic agent for multiple myeloma, a form of blood cancer previously considered incurable, as well as for mature B-cell non-Hodgkin lymphomas.
SCHLÜSSELPUBLIKATIONEN / KEY PUBLICATIONS
Liang-Ping Li, J. Christoph Lampert, Xiaojing Chen, Catarina Leitao, Jelena Popović, Werner Müller, Thomas Blankenstein (2010). Transgenic mice with a diverse human T-cell antigen receptor repertoire. Nature Medicine. doi:10.1038/nm.219.
Matthias Obenaus, Catarina Leitão, Matthias Leisegang, Xiaojing Chen, Ioannis Gavvovidis, Pierre van der Bruggen, Wolfgang Uckert, Dolores J Schendel, Thomas Blankenstein (2015). Identification of human T-cell receptors with optimal affinity to cancer antigens using antigen-negative humanized mice. Nature Biotechnology. doi:10.1038/nbt.3147.
Matthias Leisegang, Thomas Kammertoens, Wolfgang Uckert, Thomas Blankenstein (2016). Targeting human melanoma neoantigens by T cell receptor gene therapy. Journal of Clinical Investigation. doi:10.1172/JCI83465.
This electron microscope image shows T-cells (highlighted red) attacking a cancer cell.