New muscle therapy gets fast-track boost
To help bring therapies for rare muscle diseases in children to market sooner, the Berlin-based start-up MyoPax, a spin-off from the Max Delbrück Center and Charité – Universitätsmedizin Berlin, has now received a boost from the U.S. Food and Drug Administration (FDA). The company has been granted the FDA’s orphan drug designation (ODD) and rare pediatric disease designation (RPDD), both of which offer multiple regulatory and financial advantages – including fast-track approval status and, eventually, market exclusivity.
But first, the researchers must test their new therapeutic approach in a clinical trial sponsored by Charité. The German Federal Ministry of Education and Research and ForTra gGmbH of the Else Kröner-Fresenius Foundation are funding the trial, which will start with the first patients in the fall and should be completed by 2026.
The result of many years of research
The innovative muscle stem cell technology selected by the FDA is based on years of research by Professor Simone Spuler and her Myology Lab team at the Experimental and Clinical Research Center (ECRC), a joint institution of the Max Delbrück Center and Charité. Spuler founded MyoPax last year, together with Dr. Verena Schöwel-Wolf. Their goal is to develop therapies for local muscle defects, acute muscle wasting and hereditary muscular dystrophies that are currently incurable or for which adequate treatment does not yet exist.
With their stem cell therapy, the team wants to help children who suffer from exstrophy-epispadias complex (EEC). This spectrum of rare congenital disorders is characterized by the usually hollow bladder lying inverted and opened like a plate on the abdominal wall. In addition to malformations of the abdominal muscles, pelvic bones, urethra and external genitalia, the bladder sphincter also remains underdeveloped. This muscle defect is due to delayed cell migration during embryonic development and causes lifelong incontinence. About one in 11,000 children is born with EEC and the malformations are currently surgically corrected. Often, further surgery is required to improve bladder function. With the help of MyoPax’s new stem cell therapy, however, bladder sphincter muscle can be rebuilt, thus allowing the patient to regain long-term bladder control.
“Receiving this recognition from the FDA is an important milestone in our work,” says Spuler. “It confirms that our new therapeutic approach has the potential to improve the lives of young patients with EEC and other muscle diseases – as well as the lives of their parents.”
With her second company, MyoPax Denmark ApS, Spuler has been accepted into the incubator program of the BioInnovation Institute (BII) Foundation in Copenhagen, which provides financial and strategic support to help the company keep up with international competition. Meanwhile, MyoPax is preparing for expansion to the United States.
Text: Jana Ehrhardt-Joswig
Further information
Downloads
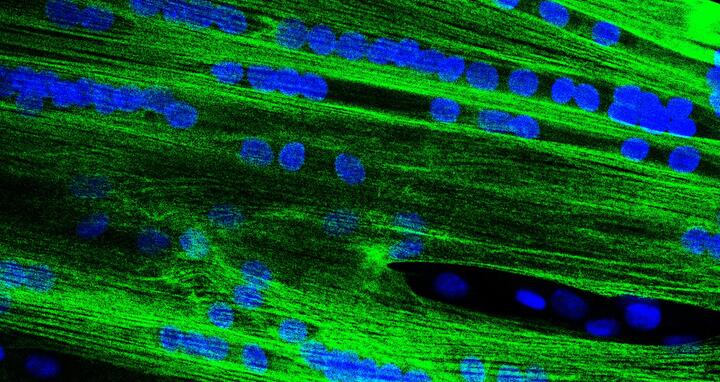
Individual muscle stem cells fuse to form multinucleated elongated muscle fibre precursors. Image: Dr. Eric Metzler, scientist at MyoPax GmbH
Contact
Dr. Verena Schöwel-Wolf
CEO of MyoPax
info@myopax.eu
Christina Anders
Redakteurin, Kommunikation
Max Delbrück Center
+49 30 9406-2118
christina.anders@mdc-berlin.de oder presse@mdc-berlin.de
The Max Delbrück Center for Molecular Medicine in the Helmholtz Association (Max Delbrück Center) is one of the world’s leading biomedical research institutions. Max Delbrück, a Berlin native, was a Nobel laureate and one of the founders of molecular biology. At the locations in Berlin-Buch and Mitte, researchers from some 70 countries study human biology – investigating the foundations of life from its most elementary building blocks to systems-wide mechanisms. By understanding what regulates or disrupts the dynamic equilibrium of a cell, an organ, or the entire body, we can prevent diseases, diagnose them earlier, and stop their progression with tailored therapies. Patients should be able to benefit as soon as possible from basic research discoveries. This is why the Max Delbrück Center supports spin-off creation and participates in collaborative networks. It works in close partnership with Charité – Universitätsmedizin Berlin in the jointly-run Experimental and Clinical Research Center (ECRC), the Berlin Institute of Health (BIH) at Charité, and the German Center for Cardiovascular Research (DZHK). Founded in 1992, the Max Delbrück Center today employs 1,800 people and is 90 percent funded by the German federal government and 10 percent by the State of Berlin.