Passenger genes allow more targeted cancer therapy
Genetic alterations are widely recognized as a leading cause of cancer. These are often amplifications involving the duplication of genes that promote cancer development, such as oncogenes and their enhancer elements. These genes are copied excessively and are then present in large numbers either within the genome or as separate DNA rings.
"Specifically, the cell not only duplicates cancer genes but also copies the preceding and succeeding sections of DNA," explains Professor Anton Henssen, head of the research group "Genomic Instability in Pediatric Tumors" at the Experimental and Clinical Research Center (ECRC). “These co-copied sections often contain additional genes, previously considered inconsequential to cancer development and thus termed “passenger genes.”
Not-so-silent passengers
If confirmed, we would have a new marker for targeted therapy selection.
Collaborating with Dr. Jan Dörr, also a pediatric oncologist at Charité and a researcher at ECRC, the scientists demonstrated that these genetic hitchhikers are more than mere silent passengers in the study published in the journal "Cancer Discovery." They disrupt fundamental processes within the cell, causing the tumor cell to become dependent on processes unrelated to tumor growth. “This creates an Achilles’ heel at a completely unexpected location that was previously unknown” remarks Henssen. “By targeting passenger genes in treatment, we can attack cancer from a new angle."
The researchers illustrated how these previously unknown dependencies could be therapeutically exploited, using neuroblastoma as an example. Neuroblastoma, a particularly aggressive cancer affecting young children, was shown to be much more susceptible to the approved cancer drug rapamycin in mice, when both the cancer gene MYCN and the passenger gene DDX1 were present in high numbers. "The passenger gene disrupts the tumor cell's metabolism,” Dörr explains. “The cell has to compensate for the disruption, and rapamycin prevents this. This ultimately leads to the death of the tumor cell."
Treating neuroblastoma with rapamycin, in addition to other drugs, could potentially benefit patients whose tumors have amplified both the cancer and passenger genes. The researchers plan to confirm this finding in clinical studies. "If confirmed, we would have a new marker for targeted therapy selection,” says Dörr.
Evident across various tumor types
Therefore, we consider the approach of targeting tumors, including their passenger genes, promising for other cancer types as well.
The potential benefits of focusing on passenger genes is likely not limited to neuroblastoma. The study, based on an analysis of millions of data points regarding gene interdependencies in 26 different tumor types, showed that in ten cases, new dependencies emerged due to passenger genes. Yi Bei, a doctoral student in Henssen's team and first author on the publication notes, "We believe this is just the tip of the iceberg, and with better data, we could discover more cases."
Anton Henssen emphasizes that the creation of vulnerabilities by passenger genes in tumors appears to be a widespread phenomenon. "Therefore, we consider the approach of targeting tumors, including their passenger genes, promising for other cancer types as well."
Text: Stefanie Reinberger, Max Delbrück Center / Romy Greiner, Charité
- About the study
The study was funded by the European Research Council (ERC) and received additional support from the "Cancer Grand Challenges" initiative, jointly supported since 2020 by Cancer Research UK and the National Cancer Institute of the National Institutes of Health in the US. It was co-led by Anton Henssen and Jan Dörr, both practicing physicians at the Department of Pediatrics at Charité with a focus on Oncology and Hematology. At the ECRC, Jan Dörr heads the junior research group "Tumor Heterogeneity and Therapy Resistance in Pediatric Tumors," while Anton Henssen leads the research group "Genomic Instability in Pediatric Tumors." Anton Henssen is supported by a Mildred-Scheel Professorship from the German Cancer Aid and is a scientific member of the German Consortium for Translational Cancer Research (DKTK) in Berlin.
- Cancer Grand Challenges
Co-founded in 2020 by two of the largest funders of cancer research in the world: Cancer Research UK and the National Cancer Institute, Cancer Grand Challenges supports a global community of interdisciplinary, world-class research teams to come together, think differently and take on some of cancer’s toughest challenges. These are the obstacles that continue to impede progress, and no one scientist, institution, or country will be able to solve them alone. With awards of up to £20M, Cancer Grand Challenges teams are empowered to rise above the traditional boundaries of geography and discipline to make the progress against cancer we urgently need.
Further information
Literature
Marieluise Kirchner et al. (2023): „Passenger gene co-amplifications create collateral therapeutic vulnerabilities in cancer“, Cancer Discovery, DOI: 10.1158/0008-5472.CAN-23-1189.
Downloads
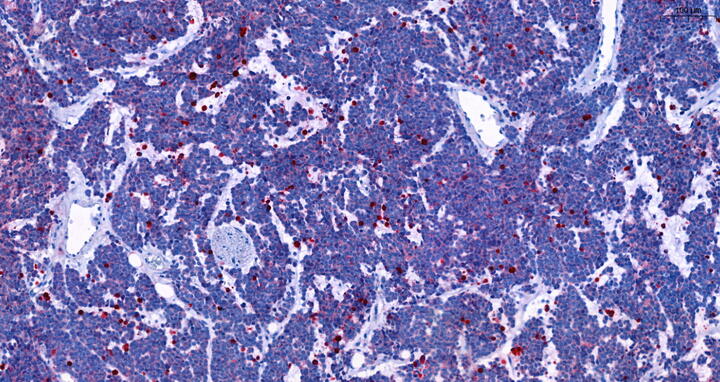
Red staining of dead cells in DDX1-MYCN co-amplified neuroblastoma after treated with rapamycin. Henssen lab, Max Delbrück Center
Contacts
Professor Anton Henssen
Department of Pediatric Oncology and Hematology
Experimental and Clinical Research Center (ECRC)
Charité – Universitätsmedizin Berlin
+49 30 450 566 132
anton.henssen@charite.de
Christina Anders
Editor, Communications
Max Delbrück Center
+49 (0)30 9406-2118
christina.anders@mdc-berlin.de or presse@mdc-berlin.de
- Max Delbrück Center
The Max Delbrück Center for Molecular Medicine in the Helmholtz Association (Max Delbrück Center) is one of the world’s leading biomedical research institutions. Max Delbrück, a Berlin native, was a Nobel laureate and one of the founders of molecular biology. At the locations in Berlin-Buch and Mitte, researchers from some 70 countries study human biology – investigating the foundations of life from its most elementary building blocks to systems-wide mechanisms. By understanding what regulates or disrupts the dynamic equilibrium of a cell, an organ, or the entire body, we can prevent diseases, diagnose them earlier, and stop their progression with tailored therapies. Patients should be able to benefit as soon as possible from basic research discoveries. This is why the Max Delbrück Center supports spin-off creation and participates in collaborative networks. It works in close partnership with Charité – Universitätsmedizin Berlin in the jointly-run Experimental and Clinical Research Center (ECRC), the Berlin Institute of Health (BIH) at Charité, and the German Center for Cardiovascular Research (DZHK). Founded in 1992, the Max Delbrück Center today employs 1,800 people and is 90 percent funded by the German federal government and 10 percent by the State of Berlin.