Third ERC Grant for Gaetano Gargiulo
Gaetano Gargiulo.
Dr Gaetano Gargiulo, head of the Molecular Oncology Lab at the Max Delbrück Center, and his team are working on a screening tool that distinguishes between cell states of CAR T cells when they are either very effective at killing cancer cells or exhausted. The European Research Council is supporting the initial steps toward commercialization with a Proof of Concept (PoC) Grant of €150,000. Gargiulo is among 240 researchers from across Europe who received such funding in last year’s three competition rounds. The ERC announced 102 PoC grants on January 18th, paving the way for the researchers to translate their pioneering findings into broadly applicable solutions.
After a Starting Grant in 2016 and a Proof of Concept Grant in 2022, this is his third ERC Grant. “I am privileged to be continuously supported by European Research Council,” says Gargiulo. “We were blessed with an ERC Starting Grant in the past and created a very flexible technology to study how cancer cells change their state to become worse. We realized that we can also use it to improve immune cells that we genetically engineer to combat cancer. This funding instrument gives us the momentum to tap into this lead.”
Some T cells fall short – for many reasons
CAR T-cell therapies are often a last resort for patients with certain types of leukemia and lymphoma who do not respond to standard treatments – and new versions of these cell-based immunotherapies are being developed for solid tumors as well. This technique involves taking immune cells (T cells) from the patient and equipping them with a chimeric antigen receptor (CAR) in the laboratory. The CAR acts like a tiny antenna, patrolling the body’s cells for specific features on the cancer cells (antigens). Once the CAR T cells are re-introduced back into the patient’s body, they begin to detect and destroy cancer cells with the antigen that fits their new receptors.
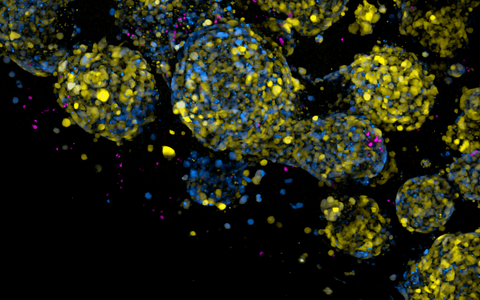
Engineered immune cells (shown as small round magenta dots) surrounding brain tumor cells with distinct identities as revealed by a dual synthetic DNA-driven fluorescent reporters (blue & yellow).
However, stumbling blocks such as the intricate manufacturing process, excessive exposure to antigens, the harsh environment within a tumor and in its immediate neighborhood can result in T cells that fall short, hampering their efficacy against both blood cancers and solid tumors. The manufacturing process itself is also very expensive, on the order of hundreds of thousands of Euros, so even minimal modifications to the process that might make it more efficient in producing effective CAR T cells could make this approach more sustainable and available to more patients.
Screening and reversing dysfunction
With the ERC Proof of Concept Grant, the Gargiulo Lab aims to create and validate a novel tool to improve the quality of T cell products in the lab. It's called SynT, a synthetic reporter system designed to indicate different cell states that either render T cells dysfunctional or that represent a potent “serial killer” mode. These cell states are detected by lab-engineered segments of DNA that switches a fluorescent protein on or off (named synthetic locus control region or sLCR). Depending on which sLCR is turned on, the cells glow in a different color when observed under a fluorescence microscope. With a fast microscope and robotic platform, the team can test hundreds of conditions in parallel, to find those that enhance the “serial killer” mode.
“This screening can help us to pinpoint signaling pathways or pharmacological agents that can boost functional CAR T cells and reverse dysfunction. SynT will help us better understand the process of cell bioengineering that underlies cell therapy for cancer, and potentially to improve the manufacturing to increase activity and reduce costs,” says Gargiulo. “Ultimately, these advances can make CAR T cell therapies even more effective.”
Further information
- ERC press release
- ERC Proof of Concept Grant for Gaetano Gargiulo
- ERC grantees at the Max Delbrück Center
Downloadable images
- Profile of Gaetano Gargiulo. Photo: David Ausserhofer, Max Delbrück Center
- Engineered immune cells (shown as small round magenta dots) surrounding brain tumor cells with distinct identities as revealed by a dual synthetic DNA-driven fluorescent reporters (blue & yellow). Photo: Matthias Jürgen Schmitt, Gargiulo Lab, Max Delbrück Center
Contacts
Dr Gaetano Gargiulo
Head of the Molecular Oncology Lab
Max Delbrück Center
+49(0)30-9406-3861
Gaetano.Gargiulo@mdc-berlin.de
Jana Schlütter
Editor, Communications
Max Delbrück Center
+49 30 9406-2121
jana.schluetter@mdc-berlin.de or presse@mdc-berlin.de
- Max Delbrück Center
The Max Delbrück Center for Molecular Medicine in the Helmholtz Association (Max Delbrück Center) is one of the world’s leading biomedical research institutions. Max Delbrück, a Berlin native, was a Nobel laureate and one of the founders of molecular biology. At the locations in Berlin-Buch and Mitte, researchers from some 70 countries study human biology – investigating the foundations of life from its most elementary building blocks to systems-wide mechanisms. By understanding what regulates or disrupts the dynamic equilibrium of a cell, an organ, or the entire body, we can prevent diseases, diagnose them earlier, and stop their progression with tailored therapies. Patients should be able to benefit as soon as possible from basic research discoveries. This is why the Max Delbrück Center supports spin-off creation and participates in collaborative networks. It works in close partnership with Charité – Universitätsmedizin Berlin in the jointly-run Experimental and Clinical Research Center (ECRC), the Berlin Institute of Health (BIH) at Charité, and the German Center for Cardiovascular Research (DZHK). Founded in 1992, the Max Delbrück Center today employs 1,800 people and is 90 percent funded by the German federal government and 10 percent by the State of Berlin.