Cryo-Electron Microscopy
Christoph Diebolder
Profil
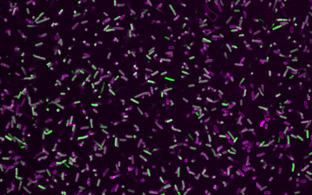
Cryo-electron microscopy (cryoEM) has become an essential tool in structural biology. It enables scientists to look at biological samples in their native state with near atomic resolution, allowing them to study the precise structure and function of complex cellular machineries such as the ribosome, the “protein producer” of the cell. More recently, a related method, cryo-electron tomography (cryoET), has been gaining attention as it allows these structures to be observed in their cellular environment.
Team
Group Leader
Research
Flash-frozen preparations in their natural structures
In order to perform cryoEM, biological samples are cooled so quickly that ice crystals cannot form. Instead, glass-like water forms, tightly enclosing the molecules in their natural state. The electron beam of the microscope can penetrate the amorphous layer. A camera installed in the lower part of the microscope records the image and a computer calculates an exact three-dimensional image of the molecule from the often hundreds of thousands of photos. For conventional electron microscopy, samples must first be dehydrated, chemically fixed and stained with heavy metals to enhance image contrast. Consequently, scientists have to interpret artefacts. The cryoEM, on the other hand, makes it possible to analyse individual molecules in their native aqueous environment and observe how they interact with each other. This way, CryoEM closes a gap in biological structure elucidation.
The team at the core facility – a joint project of the Charité – Universitätsmedizin Berlin, the Max Delbrück Center for Molecular Medicine (MDC) as well as the Leibnitz Research Institute for Molecular Pharmacology (FMP) – has implemented a special tomography workflow: First, the scientists make the sample carriers hydrophilic by glow-discharging in a plasma. Then, they mark the areas of the carriers on which the target cells are to grow. To estimate when is the best time to flash-freeze the cells they use live cell imaging. In a next step, they identify the areas in the cells that should be imaged by confocal cryo-fluorescence light microscopy. They then mill these out using a focused ion beam (FIB). Finally, they can image these cell lamellas in high resolution three dimensions by tomography using a Titan Krios cryo-transmission electron microscope. In addition to tomography, this microscope is also suitable for single particle analysis, a technique that allows structural analysis of biological samples at near atomic resolution.
To move from in vitro to in situ
After construction in 2019 and2020, the facility opened its doors for users in spring 2021. Initial tests indicate very high performance of the outmost sensitive core instrumentation. The team of scientists maintaining and operating the facility – Metaxia Stavroulaki, Thiemo Sprink, and Christoph Diebolder, who is heading the facility – aims to provide the users with data of highest quality.
Future developments in the facility will focus on streamlining the new and challenging in-situ cryo-electron tomography workflow. “We would like our work to advance structural biology in a methodical way,” says Diebolder, describing his vision. “Our goal is to move from in vitro to in situ, that is, to observe biological processes directly in the cell.”